Background
Although efficacy and safety data on chimeric antigenic receptor (CAR) T-cell therapy for patients aggressive B-cell lymphomas are being accumulated, there is limited research on health-related quality of life (HRQoL) and symptom burden of these patients. Additionally, the few HRQoL data available mainly stems from selected patients enrolled in clinical trial settings.
Objective
The primary objective of this study was to compare HRQoL of patients with relapsed/refractory aggressive B-cell lymphomas just before receiving CAR T-cell infusion in real-life, with that of their peers in the general population. Secondary objectives were to describe the prevalence of disease-specific symptoms and worries, and to investigate the relationships between symptom burden and HRQoL and functional outcomes.
Methods:
Baseline data from an ongoing prospective observational study by the Italian GIMEMA Group were analyzed. Inclusion criteria were: adult patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL transformed by indolent lymphoma and mantle cell lymphoma, scheduled to receive CAR T-cell therapy.
HRQoL was assessed with the EORTC QLQ-C30 and its recently validated module for patients with high-grade non-Hodgkin lymphoma (EORTC QLQ‐NHL‐HG29). Multivariable linear regression analyses were performed to estimate the overall mean difference in the QLQ-C30 scores between patients and the general population, adjusting for age, sex and comorbidity. Evidence‐based guidelines for interpretation of the QLQ‐C30 were used to determine clinically meaningful differences between groups.
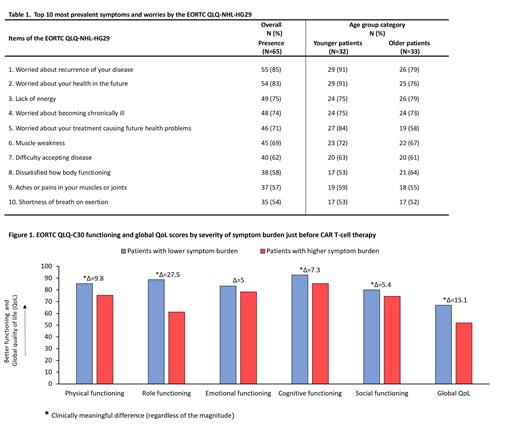
The prevalence of patients who reported symptoms and worries on the QLQ‐NHL‐HG29 was based on the number of patients who answered, “a little,” “quite a bit,” or “very much” on a given item. This was assessed overall and by age groups. For descriptive purposes, we divided the sample by the median age, deriving two subgroups: younger (with a median age of 50 years; IQR: 41 - 56) and older patients (with a median age of 65 years; IQR: 63 - 71). Mean scores of functional and global QoL scales of the EORTC QLQ-C30 were analyzed by severity of symptom burden. Patients were categorized as having high or low symptom burden based on the median score of the validated symptom burden scale of the QLQ‐NHL‐HG29 questionnaire.
Results
Overall, 65 patients enrolled across 9 centers were analyzed. Median age at study entry was 61 years (IQR, 50-65) and 21 (32%) were women. Median time since diagnosis was 2 years (IQR, 1-4). Eighteen patients (28%) had already received autologous hematopoietic stem cell transplantation (auto-HSCT) before study inclusion. Median number of previous lines of therapy was 2 (IQR: 2-3), and eleven patients (18%) reported at least 1 comorbidity.
Patients reported statistically and clinically meaningful worse scores across several domains, compared with their peers in the general population. The top three largest statistically and clinically meaningful differences with regard to functional scales were observed for role functioning (Δ=26.8, p<.001), social functioning (Δ=17.6, p<.001) and the global QoL scale (Δ=14.3, p<.001). With regard to symptoms, the two largest statistically and clinically meaningful worse symptoms, compared to the general population, were observed for fatigue (Δ=14.2, P<.001), dyspnea (Δ=10.9, p=.003) and financial difficulty (Δ=10.6, p=.001). Prevalence of symptoms and worries was high with more than 50% of patients reporting 14 different symptoms and worries. Younger patients tended to report a higher prevalence in regard to specific worries. The top ten most prevalent symptoms and worries by the QLQ‐NHL‐HG29, overall and by age groups, are reported in Table 1.
Patients with a greater symptom burden had a clinically meaningful worse global QoL, and worse physical, role, social, and cognitive functioning compared to those with a lower symptom burden (Figure 1).
Conclusion:
Using a validated disease-specific measure for high-grade non-Hodgkin lymphoma, we observed that just before CAR T-cell therapy in real-life, patients report substantial HRQoL impairments and a high prevalence of symptoms and worries. Our findings also suggest to pay special attention to patients with a greater symptom burden, being a vulnerable group with important HRQoL and functional impairments.
Disclosures
Efficace:AbbVie: Consultancy; Incyte: Consultancy; Syros: Consultancy. Zinzani:SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Bonifazi:Sanofi: Honoraria; Neovii: Honoraria. Chiappella:Gilead-Sciences: Other: lecture fee/educational activities, advisory board; Roche: Other: lecture fee/educational activities, advisory board; Ideogen: Other: advisory board; Takeda: Other: lecture fee/educational activities, advisory board; AstraZeneca: Other: lecture fee/educational activities; Incyte: Other: lecture fee/educational activities; Jannsen-Cilag: Other: lecture fee/educational activities, advisory board; Novartis: Other: lecture fee/educational activities; Celgene-BMS: Other: lecture fee/educational activities, advisory board; SecuraBIO: Other: advisory board. Russo:Medac, Abbvie, MSD, Jazz Pharma, Gilead, Novartis: Membership on an entity's Board of Directors or advisory committees; MSD, Novartis, Gilead, BMS, Medac: Honoraria. Botto:Takeda: Speakers Bureau. Corradini:Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations. Vignetti:Novartis: Speakers Bureau; AbbVie: Honoraria; Uvet: Honoraria; Dephaforum: Honoraria; ER Congressi: Honoraria; IQVIA: Honoraria. Di Rocco:Takeda: Speakers Bureau; Abbvie: Honoraria; Janssen: Honoraria; Gilead: Honoraria, Speakers Bureau; Novartis: Speakers Bureau; Roche: Honoraria, Speakers Bureau; Incyte: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal